WEBINAR RECORDING
Advancements in 3D modelling : Building mature, functional 3D skeletal muscle microtissues in vitro.
In this webinar, Dr. Marieke Aarts from Bi/ond and Amanda Turner from bit.bio will explain how Bi/ond has successfully cultured bit.bio’s human iPSC-derived ioSkeletal Myocytes™ on the perfusable organ-on-chip platform, generating 3D skeletal muscle microtissues.
They will also examine how functional 3D muscle bundles containing striated multinucleated myocytes can be reliably generated in less than a week.
In addition, they will discuss how to pace and train muscle tissue simulating physiological conditions, and how to achieve accelerated muscle maturation and enhanced contractile response.
Finally, they will share how Bi/ond’s chip technology can assist with the study of vascularization, dynamic drug response, and even mimic the immune system's response.
Access this on-demand webinar if you want to:
-
Discover how to culture 3D muscle cell bundles on Bi/ond's MUSbit™ platform.
-
Learn how to combine Bi/ond's technology with ioSkeletal Myocytes for measuring muscle contractions triggered by electrical stimulation
-
Explore the translational potential of the MUSbit/Let-it-bit™ for pharmacological studies on wild-type and disease model cells.
This webinar was originally presented live on 13th October 2023. If you missed it then, do not waste the possibility to access it now!
Why does it matter?
The MUSbit™ is a perfusable organ-on-chip containing two pillars that serve as anchoring points for your muscle cell bundles. We have now gone one step further, integrating sensors (we call this new generation of chips Let-it-bit™).
Take your research to the next level by combining your tissue with a microfluidic channel! This integration allows you to study vascularization, dynamic drug response, and even mimic the immune system's response.
Furthermore, unlike other systems, the MUSbit ™ allows you to create 3D tissues with a limited number of cells, saving your budget without compromising the quality of your research.
In combination with integrated electrodes, you can pace and train your muscle tissue simulating real physiological conditions and achieve accelerated muscle maturation and enhanced cell stimulation, significantly advancing your research.
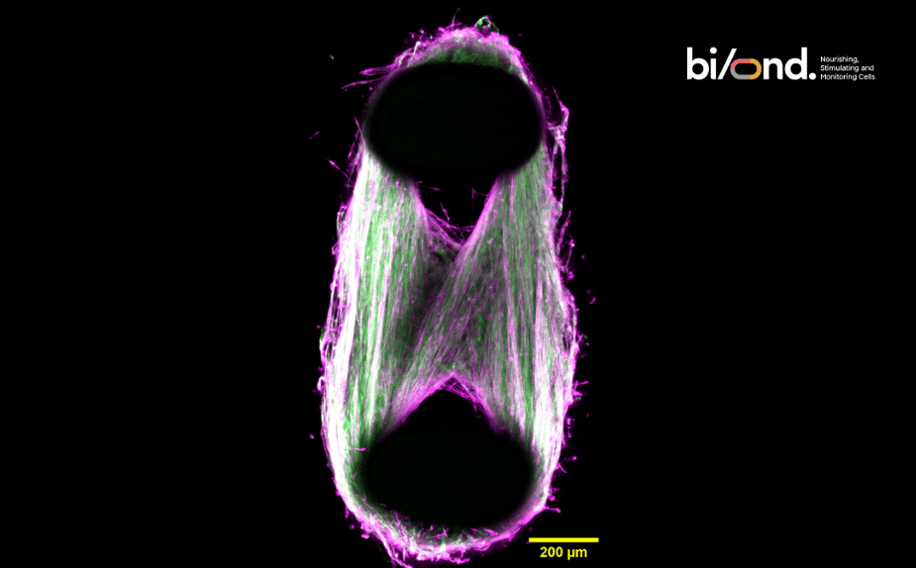
Our groundbreaking microphysiological system establishes a path towards creating more physiologically relevant biomimetic models for advancing drug development and disease modeling

